10 mind-boggling things you should know about quantum physics
From the multiverse to black holes, here’s your cheat sheet to the spooky side of the universe.
1. The quantum world is lumpy

The quantum world has a lot in common with shoes. You can’t just go to a shop and pick out sneakers that are an exact match for your feet. Instead, you’re forced to choose between pairs that come in predetermined sizes.
The subatomic world is similar. Albert Einstein won a Nobel Prize for proving that energy is quantized. Just as you can only buy shoes in multiples of half a size, so energy only comes in multiples of the same "quanta" — hence the name quantum physics.
The quanta here is the Planck constant, named after Max Planck, the godfather of quantum physics. He was trying to solve a problem with our understanding of hot objects like the sun. Our best theories couldn’t match the observations of the energy they kick out. By proposing that energy is quantized, he was able to bring theory neatly into line with experiment.
2. Something can be both wave and particle
J. J. Thomson won the Nobel Prize in 1906 for his discovery that electrons are particles. Yet his son George won the Nobel Prize in 1937 for showing that electrons are waves. Who was right? The answer is both of them. This so-called wave-particle duality is a cornerstone of quantum physics. It applies to light as well as electrons. Sometimes it pays to think about light as an electromagnetic wave, but at other times it’s more useful to picture it in the form of particles called photons.
A telescope can focus light waves from distant stars, and also acts as a giant light bucket for collecting photons. It also means that light can exert pressure as photons slam into an object. This is something we already use to propel spacecraft with solar sails, and it may be possible to exploit it in order to maneuver a dangerous asteroid off a collision course with Earth, according to Rusty Schweickart, chairman of the B612 Foundation.
3. Objects can be in two places at once

Wave-particle duality is an example of superposition. That is, a quantum object existing in multiple states at once. An electron, for example, is both ‘here’ and ‘there’ simultaneously. It’s only once we do an experiment to find out where it is that it settles down into one or the other.
This makes quantum physics all about probabilities. We can only say which state an object is most likely to be in once we look. These odds are encapsulated into a mathematical entity called the wave function. Making an observation is said to ‘collapse’ the wave function, destroying the superposition and forcing the object into just one of its many possible states.
This idea is behind the famous Schrödinger’s cat thought experiment. A cat in a sealed box has its fate linked to a quantum device. As the device exists in both states until a measurement is made, the cat is simultaneously alive and dead until we look.
4. It may lead us towards a multiverse

The idea that observation collapses the wave function and forces a quantum ‘choice’ is known as the Copenhagen interpretation of quantum physics. However, it’s not the only option on the table. Advocates of the ‘many worlds’ interpretation argue that there is no choice involved at all. Instead, at the moment the measurement is made, reality fractures into two copies of itself: one in which we experience outcome A, and another where we see outcome B unfold. It gets around the thorny issue of needing an observer to make stuff happen — does a dog count as an observer, or a robot?
Instead, as far as a quantum particle is concerned, there’s just one very weird reality consisting of many tangled-up layers. As we zoom out towards the larger scales that we experience day to day, those layers untangle into the worlds of the many worlds theory. Physicists call this process decoherence.
5. It helps us characterize stars
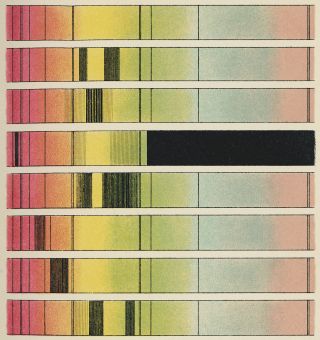
Danish physicist Niels Bohr showed us that the orbits of electrons inside atoms are also quantized. They come in predetermined sizes called energy levels. When an electron drops from a higher energy level to a lower energy level, it spits out a photon with an energy equal to the size of the gap. Equally, an electron can absorb a particle of light and use its energy to leap up to a higher energy level.
Astronomers use this effect all the time. We know what stars are made of because when we break up their light into a rainbow-like spectrum, we see colors that are missing. Different chemical elements have different energy level spacings, so we can work out the constituents of the sun and other stars from the precise colors that are absent.
6. Without it the sun wouldn’t shine

The sun makes its energy through a process called nuclear fusion. It involves two protons — the positively charged particles in an atom — sticking together. However, their identical charges make them repel each other, just like two north poles of a magnet. Physicists call this the Coulomb barrier, and it’s like a wall between the two protons.
Think of protons as particles and they just collide with the wall and move apart: No fusion, no sunlight. Yet think of them as waves, and it’s a different story. When the wave’s crest reaches the wall, the leading edge has already made it through. The wave’s height represents where the proton is most likely to be. So although it is unlikely to be where the leading edge is, it is there sometimes. It’s as if the proton has burrowed through the barrier, and fusion occurs. Physicists call this effect "quantum tunneling".
7. It stops dead stars collapsing

Eventually fusion in the sun will stop and our star will die. Gravity will win and the sun will collapse, but not indefinitely. The smaller it gets, the more material is crammed together. Eventually a rule of quantum physics called the Pauli exclusion principle comes into play. This says that it is forbidden for certain kinds of particles — such as electrons — to exist in the same quantum state. As gravity tries to do just that, it encounters a resistance that astronomers call degeneracy pressure. The collapse stops, and a new Earth-sized object called a white dwarf forms.
Degeneracy pressure can only put up so much resistance, however. If a white dwarf grows and approaches a mass equal to 1.4 suns, it triggers a wave of fusion that blasts it to bits. Astronomers call this explosion a Type Ia supernova, and it’s bright enough to outshine an entire galaxy.
8. It causes black holes to evaporate

A quantum rule called the Heisenberg uncertainty principle says that it’s impossible to perfectly know two properties of a system simultaneously. The more accurately you know one, the less precisely you know the other. This applies to momentum and position, and separately to energy and time.
It’s a bit like taking out a loan. You can borrow a lot of money for a short amount of time, or a little cash for longer. This leads us to virtual particles. If enough energy is ‘borrowed’ from nature then a pair of particles can fleetingly pop into existence, before rapidly disappearing so as not to default on the loan.
Stephen Hawking imagined this process occurring at the boundary of a black hole, where one particle escapes (as Hawking radiation), but the other is swallowed. Over time the black hole slowly evaporates, as it’s not paying back the full amount it has borrowed.
9. It explains the universe’s large-scale structure

Our best theory of the universe’s origin is the Big Bang. Yet it was modified in the 1980s to include another theory called inflation. In the first trillionth of a trillionth of a trillionth of a second, the cosmos ballooned from smaller than an atom to about the size of a grapefruit. That’s a whopping 10^78 times bigger. Inflating a red blood cell by the same amount would make it larger than the entire observable universe today.
As it was initially smaller than an atom, the infant universe would have been dominated by quantum fluctuations linked to the Heisenberg uncertainty principle. Inflation caused the universe to grow rapidly before these fluctuations had a chance to fade away. This concentrated energy into some areas rather than others — something astronomers believe acted as seeds around which material could gather to form the clusters of galaxies we observe now.
10. It is more than a little ‘spooky’

As well as helping to prove that light is quantum, Einstein argued in favor of another effect that he dubbed ‘spooky action at distance’. Today we know that this ‘quantum entanglement’ is real, but we still don’t fully understand what’s going on. Let’s say that we bring two particles together in such a way that their quantum states are inexorably bound, or entangled. One is in state A, and the other in state B.
The Pauli exclusion principle says that they can’t both be in the same state. If we change one, the other instantly changes to compensate. This happens even if we separate the two particles from each other on opposite sides of the universe. It’s as if information about the change we’ve made has traveled between them faster than the speed of light, something Einstein said was impossible.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Colin Stuart is an award-winning astronomy author, speaker and tutor based in the UK. His popular science books have sold over 400,000 copies worldwide and have been translated into 21 languages. The asteroid (15347) Colinstuart is named after him and he runs an online Astrophysics for Beginners course and a science writing course.
